Vaccination for Staff and Research Degree Students
Staff and Research Degree Students who handle human or animal blood, tissue, excrement or body fluids, soil, unaged compost, pathogenic bacteria, prions, parasites or viruses are often at risk of occupational exposure to vaccine-preventable diseases. Personnel considered at high risk are those in direct physical contact, contact with contaminated surfaces/equipment or exposed to infection by the respiratory route.
Immunisation is available to staff and Research Degree Students. The Communicable Diseases and Immunisation Guidelines (260kb) provides information on current recommended vaccinations and procedures.
Research Group Leaders must conduct a risk assessment as part of their IBC application to use biologicals. The IBC will recommend vaccinations to be undertaken.
Vaccination is also available for technical and professional staff at risk of occupational exposure to vaccine preventable diseases. Line Managers should complete a Technical and Professional Staff Risk Assessment Form to determine the need for vaccination of technical and professional staff.
Staff and Research Degree students requiring vaccination should be given a vaccination form to take to either the UniSA Health Medical Clinic or GP. Personnel will return the completed form to their Line Manager for record keeping on the secure University servers.
Cost of vaccination can be charged directly to either Academic Unit, Institute, Centre, Portfolio or research project funding, through a UniSA Health Medical Clinic. To claim on this system a fs32 form must be completed and submitted to the UniSA Health Medical Clinic. Personnel may use their own General Practioner or Travel Doctor and then seek reimbursement through their Research Group Leader or Facility Manager.
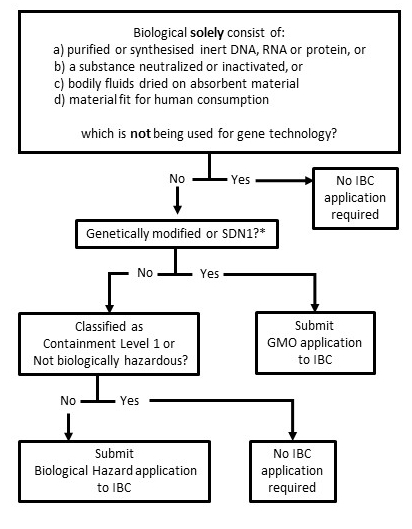
A schematic representation of processes (600kb) is available. Further information is available through the Biosafety Officer, biosafety@unisa.edu.au.